What is an Oxidant?
Oxidants are generated by our own body as a result of the natural process of aerobic metabolism, introduced from environment, or produced in our body prompted by external insults. By far the largest contribution comes from our own body as a byproduct of the energy generating reactions within mitochondria.
We owe our physical existence to mitochondria that are present in every cell of our body. Their numbers range from hundreds to thousands per cell. Mitochondria are �battery factories� in a cell where the fuels of carbohydrates, fats and proteins are brought in and converted into the molecular batteries called Adenosine Triphosphate (ATP), which are then carried out of mitochondria for use. We draw energy from the ATP molecules as needed.
A mitochondrion is enclosed by two layers of membrane with the inner membrane being of mitochondrial origin and the outer membrane of our own (Mitochondria are thought to be aerobic bacteria incorporated into our ancestor cells in endosymbiosis about 1.5 billion years ago). The outer membrane is readily permeable to small molecules and ions, but the inner membrane is impermeable to those, including protons (H+). However, specific transporters present in the intermembrane space make it possible for enzymes and nutrients to cross the inner membrane into the mitochondrial matrix, and for the ATP molecules, the finished product, to be brought out of the matrix.
In the mitochondrial matrix where oxidation of nutrients, the first step in energy generation, takes place, all the enzymatic machinery necessary for the reactions is in place. Some of the enzymes are produced by mitochondria themselves using their own DNA, and some are provided from outside produced by the nuclear DNA.
As nutrients undergo oxidative breakdown, electrons are generated in the mitochondrial matrix. The electrons are then transferred on to the inner membrane of the mitochondrion and drawn within the membrane by the protein complexes that are installed in the same membrane in the order of increasing electron affinity. At the end of this chain of protein complexes is an oxygen molecule that has the highest electron affinity. The electron flow completes its circuit when the electrons are finally drawn to the oxygen molecule upon which the latter is reduced to water.
This electron flow, equivalent to electricity, represents energy. The mitochondrion uses this energy to pump protons (H+) out of the matrix against gradient into the intermembrane space. The protons accumulated in the intermembrane space creat a proton pressure across the impermeable inner membrane, much like a hydroelectric dam. As the protons spontaneously flow back into the matrix through a specialized channel, the energy of the proton flow is captured into production of ATP molecules.
This entire process is called cell respiration. This is in fact the respiration of mitochondria, which we identify as our own. We breathe in oxygen so mitochondria can respire for both of us. They adjust their respiratory activities according to the body�s need for ATP molecules. ATP depletion leads to rapid mitochondrial respiration, which in turn translates into rapid breathing by the body. Dyspnea (air hunger) is a sense we experience when oxygen supply cannot meet mitochondrial demand.
In nature nothing is perfect. Mistakes can occur in the cell respiration, too. An electron can leak out of its pathway to be picked up by oxygen. An electron can be mistakenly transferred to oxygen out of order. Or during the final stage of electron transfer, an oxygen molecule can be prematurely let go before it has received all four electrons as it is supposed to. An outcome of these mistakes is oxygen molecules with an unpaired electron.
An atom has layers of electrons in orbit, each with a maximal capacity for two electrons. An orbital electron not only revolves around the nucleus of an atom but also spins around its own axis. The spin may be either clockwise or counterclockwise. In an atom or molecule with an even number of electrons, spins are paired; that is, for every electron spinning clockwise, there is another one spinning counterclockwise. This state is associated with a high degree of chemical stability. In an atom or molecule with an odd number of electrons, there is one odd electron for which there is no other electron that counterbalance it; this is an unpaired electron.
An atom or molecule with an unpaired electron can bond with another such molecule by sharing each other�s unpaired electron. However, when an unpaired electron is not fixed in such a bond formation (�free�), the atom or molecule that harbors it is highly unstable and therefore reactive. These are called free radicals. They rob any molecule they encounter of a loosely attached electron, themselves becoming stable. Rendered unstable, the molecule robbed of an electron, in turn, seeks out an electron from another molecule further down. This chain of events leads to broken chemical bonds and chemically altered molecules, ultimately manifesting as a biological damage. This damage, however, is not irreversible. Many molecules eventually repair themselves and recover the ability to function normally. When the free radical reacts with oxygen, however, an organic peroxide is produced. This is a non-restorable form of the molecule and the damage is permanently fixed on it.
Free radicals attack nucleic acids in DNA, amino acids in proteins or double bonds in polyunsaturated fatty acids, generating widely different forms of free radicals. For biological systems, oxygen-containing radicals are the most important and referred to as Reactive Oxygen Species (ROS).
Of all the macromolecules in the body, the most vulnerable to free radical attacks are polyunsaturated fatty acids. This is because the methylene groups adjacent to an unsaturated carbon bond (-C=C-CH2-) or in between two unsaturated carbon bonds (-C=C-CH2-C=C-) have a weak carbon-hydrogen bond, making them an easy target for free radicals. As the number of double bond in polyunsaturated fatty acids increases, their rate of peroxidation increases exponentially.
Polyunsaturated fatty acids are most abundant in cell membranes, including all the membranes that segregate and protect cell organelles from the rest of the cytoplasm, as well as the cytoplasmic membranes themselves. The nuclear membranes, for example, protect DNA inside, while the mitochondrial membranes protect all the machinery and mechanisms involved in the cell respiration. Lipid peroxidation, therefore, can have detrimental effects on various aspects of cellular function.
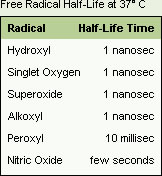
What makes lipid peroxidation all the more damaging is the fact that many harmful aldehydes are formed as a byproduct of unsaturated fatty acid oxidation. Because of their high reactivity, free radicals are extremely short-lived molecules that exert only local effects (see the Table). They are so reactive they react with the first few molecules they bump into. Aldehydes, on the other hand, have longer half-lives and have the potential to diffuse from their site of origin to reach distant intracellular and extracellular targets, thereby amplifying the damaging effects of free radicals.
Copyright 2006 Kuma.us