Oxidants in Daily Life and Common Health Conditions
Ultraviolet and X-raysBoth ultraviolet rays and X-rays are a form of energy just like heat is a form of energy. However, their energy comes in packets called photons instead of a diffuse form of heat. These rays therefore can be thought of as streams of packets of photons proceeding in a wave-like movement. The amount of energy contained in a photon increases as the wavelength of the ray decreases. X-rays have a wavelength shorter than 1/1000000 cm and have much higher energy than ultraviolet rays, whose wavelength range from 200 nm to 380 nm.
When a molecule is irradiated with ultraviolet rays, the rays' energy is deposited in the molecule, causing excitation and shift of electrons within the molecule. This electron shift can result in the formation of an unpaired electron within the molecule, turning it into a free radical.
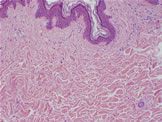
Sun exposure therefore consumes antioxidants in skin tissue. The sun-damaged skin under microscope typically shows swollen or fragmented elastic and collagen fibers in the dermis as deep as one half to two thirds of its thickness from the surface (Fig. 5). This explains the loss of elasticity seen in sun-damaged skin.
DNA molecules in the skin cells are also excited by ultraviolet energy, resulting in covalent bonding between adjacent nucleic acid bases. Altered DNA lead to mutations, which are behind the development of skin cancers.
The range of UV wavelength is often subdivided into UVA (380 � 315 nm), UVB (315 � 280 nm) and UVC (< 280 nm). The sun emits ultraviolet radiation in UVA, UVB and UVC bands, but because of the absorption in the atmosphere�s ozone layer, 99 % of the ultraviolet radiation that reaches the Earth�s surface is UVA and the remainder is UVB. UVC is completely blocked by the ozone layer.
UVA, because of its long wavelength, can penetrate clouds and glasses, and also penetrate deeper into the skin dermis. Since UVA does not cause sunburn or redness of the skin, there are no clinical signs by which it can be detected. Therefore SPF testing for sunscreens is not applicable to UVA. UVA, however, is the prime cause of wrinkles and skin ageing because it can penetrate deeper into the dermis damaging collagen and elastic fibers.
UVB with shorter wavelengths can only penetrate the epidermis, the epithelial layer that covers the skin surface. It has higher energy than UVA and makes its presence known by causing sunburn and redness of the skin, helping with the SPF testing. UVB is more linked to skin cancers than UVA. Therefore, most of the skin cancers arise from the cells within the epidermis. UVB is strongest during midday while UVA remains more or less constant from the sunrise to sunset.
More than just exciting electrons as with ultraviolet rays, X-rays with much higher energies eject electrons from irradiated molecules. The loss of an electron results in ionization of the irradiated molecule. This molecule is also a free radical because it is left with an unpaired electron. Because they ionize the irradiated molecules, X-rays are also called ionizing radiation. Ion radicals thus created have an extremely short lifetime, quickly reacting with a water molecule to produce the highly reactive Hydroxyl radicals. It is mostly through Hydroxyl radicals that X-rays damage DNA in mammalian cells, including the tumor cells targeted in radiotherapies.
We are subjected to a constant field of ionizing radiation in the form of cosmic rays as well as radiation emitted from radioactive elements, such as radium, plutonium, uranium and radon. X rays used in medical and dental examination, and radiation therapy for cancer and other diseases are another form of ionizing radiation. It is estimated that UV and ionizing radiations are responsible for about 10 % of all DNA damage caused by environmental agents.
Copyright 2006 Kuma.us